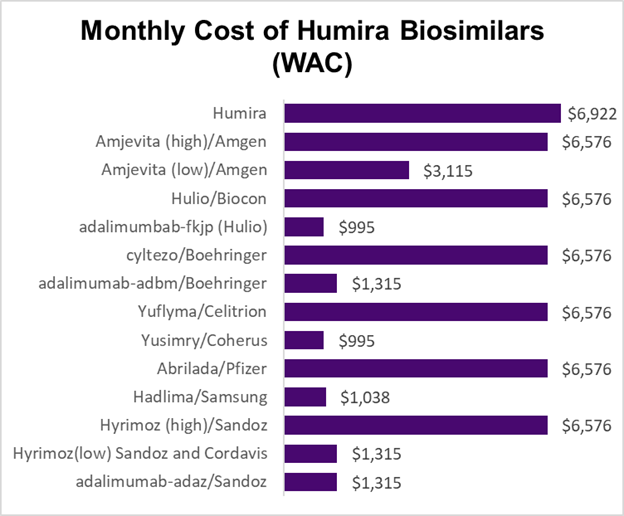
Source: Fein, Drug Channels January 9, 2024 LINK (WAC is wholesale acquisition cost, which is a “gross” cost that does not include discounts or rebates)
Biologic drugs have dramatically changed the lives of many with severe chronic inflammatory conditions, including Crohn’s Disease, Ulcerative Colitis, Rheumatoid Arthritis, Psoriasis, and Multiple Sclerosis. These drugs are manufactured by living cells - so the manufacturing is more expensive than for typical pills. Most biologic drugs are given by injection.
Biologic drugs are very expensive. So expensive that for most employees these drugs often represent a third or more of the total cost of all outpatient drugs.
Biosimilar drugs are like generics for these biologic drugs. Traditional generics, though, must be chemically entirely identical, whereas biosimilars can have very slight differences that are not clinically important. The Food and Drug Administration requires rigorous clinical testing of biosimilars to be sure that they will have the same effects as the original brand name biologic drug.
Many biosimilar medications are not interchangeable, so doctors will have to specify the biosimilar, unlike generics where a pharmacist can substitute without an explicit physician order. Biosimilar drug use could lead to $133 billion in savings by next year, but only if these drugs are really substituted for the brand name biologic, and only if the lowest cost biosimilars gain traction. Biosimilar uptake is much higher Europe, due to flexible approval processes, more oversight of patents, and price regulations.
Here is the wrinkle that might restrain savings from biosimilar medications. Many biosimilar companies are introducing both high- and low-cost biologics, and in some instances pharmacy benefit managers are promoting the use of the higher cost biologics, which often come with high rebates. The prices in the figure above do not consider rebates. Member cost share is based on the acquisition price, and the rebates can be used by the employer to lower premium cost for all members. I think of this as “reverse Robin Hood,” where those with substantial medical needs subsidize those who do not take these medications.
Some portion of the total discount from the higher cost medications might also be retained by the PBM.
Implications for employers:
Employers can talk to their pharmacy consultant and PBM to determine the financial impact of formulary choices for biosimilar drugs.
Choosing the lower priced biosimilars, which are clinically equivalent to the brand name or higher priced biosimilar, will lead to both lower overall total costs and will lead to less financial toxicity for those who are prescribed these medications.
Review PBM reports carefully to evaluate the total cost, as well as the cost per patient for available biologic medications.
Thanks for reading. You can find previous posts in the Employer Coverage archive
Please “like” and suggest this newsletter to friends and colleagues. Thanks!
Tomorrow: Paternity leaves - and the new WTW Leave Survey