COVID-19 updates, and increase in late stage cervical cancer
August 26
Today’s note is COVID-19 focused, with a note on a worrisome increase in late stage cervical cancer.
1. We know more about fall COVID-19 booster shots
The United Kingdom approved Omicron booster shots last week - these are booster shots by Pfizer and Moderna which target both the original COVID-19 strain and the BA.1 variant. The manufacturers report that this provides good neutralizing antibodies against BA.4 and BA.5, which are now predominant across the globe. The US Food and Drug Administration has instructed both manufacturers to manufacture mRNA vaccines that are instead targeted to BA.4 and BA.5. Each has already started clinical trials and is manufacturing these new vaccines at scale; they will be approved based on antibodies measured by blood tests, not on clinical proof of decreased infection rates.
Both Pfizer and Moderna have stated that they are likely to have the vaccine ready for distribution in September, so boosters will likely be available around the same time influenza vaccines are usually given. Most Americans who are now “up to date” on vaccination got their first or second booster well over 4 months ago, so this booster is likely to be recommended for at least all adults.
Implications for employers:
- Employees will be able to get both flu and COVID-19 booster vaccines this fall. They are able to get both vaccinations at the same time (in different arms).
- Giving both vaccines is preferred, as this will likely lead to higher vaccination rates.The federal government will continue to distribute the COVID-19 boosters without charge this fall (but see below).
- Both COVID-19 and influenza vaccines should be provided to members of employer sponsored health plans without out of pocket costs.
- Both COVID-19 boosters and influenza vaccines will likely be widely available at pharmacies and physician offices, although some employers are gearing up for influenza vaccine clinics.
2. Federal government will transition costs of COVID-19 vaccines and medications to employer sponsored health insurance plans
Congress has not passed legislation to provide additional funding for pandemic response, and federal funds to pay for COVID vaccinations and therapeutics are running dry. The Wall Street Journal reports that the federal government will transition these costs to employer sponsored health insurance plans (as well as Medicare and Medicaid) over the coming months.
Because the fragmented private purchasing market will likely secure these vaccines and medications at higher prices than those paid by the federal government, this change is likely to further push up health care costs at a time where many signals already suggest high premium increases over the next few years. The mechanism to pay for vaccinations and treatment for the uninsured is not yet clear. This transition from federal funding could decrease US vaccination rates, already among the lowest among developed countries.
Implications for employers:
- This is one more reason to expect higher premiums and costs for health insurance in 2023 and beyond
- Employers should be cautious about implementing prior authorization or other programs that would decrease access to COVID-19 medications, as administration of these medications is very time sensitive. In addition, there have been large changes in effectiveness of different monoclonal antibodies with each variant. Decisions to restrict vaccines to a single brand could lead to access difficulties.
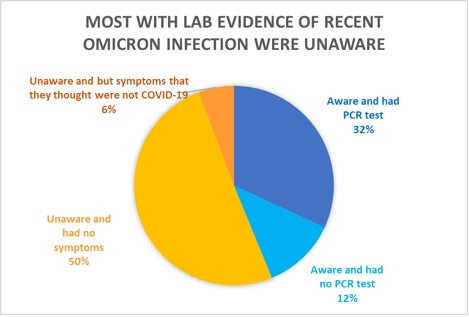
3. Majority of those with recent COVID-19 reported they were unaware
Researchers identified 210 people who showed laboratory evidence that they had recent infection with Omicron, and asked them whether they remembered any symptoms, and whether they knew they had COVID-19.
Source: Joung SY,et al JAMA Netw Open. August 17, 2022 LINK
Implications for employers:
- Many with COVID-19 may be unaware when they are contagious
- This increases the importance of improving indoor air quality.
- Individuals who are at higher risk or wish to increase their protection can gain additional protection by wearing high quality well-fitted masks.
4. Stage 4 cervical cancer rates increasing in the US
The Human Papilloma Virus (HPV) vaccine should prevent the vast majority of cases of cervical cancer, and many cases of head and neck cancer in middle age and older adults. The vaccine has been available since 2006, and about 75% of teenagers have received it, although under 60% have had both (or all three) recommended doses. Australia, which has achieved higher HPV vaccination rates with a national campaign, has already seen a steep drop in precancerous cervical findings in every age group that has been vaccinated.
Researchers in the US have found that cases of Stage 4 cervical cancer (17% five year survival) have been rising about 1.3% a year in the United States from 2001-2019. Rates of this cancer are higher among Black and Hispanic women, although the rate of increase in cancer is higher in White women. Even though pap smears or equivalent tests are now only recommended every 3-5 years, more women are behind on their screening. This data was collected before the pandemic, which dramatically decreased cancer screening.
Implications for employers:
- Cancer screening remains important, and employers should require health plans to report on screening rates for cervical, breast and colorectal cancer
- The HPV vaccine, ideally given at ages 11-12, can prevent most cases of cervical cancer, and should be used more widely. Employer pressure can encourage more health plans to report on pediatric vaccination rates.