FDA approves self-swab for cervical cancer screening
June 5, 2024
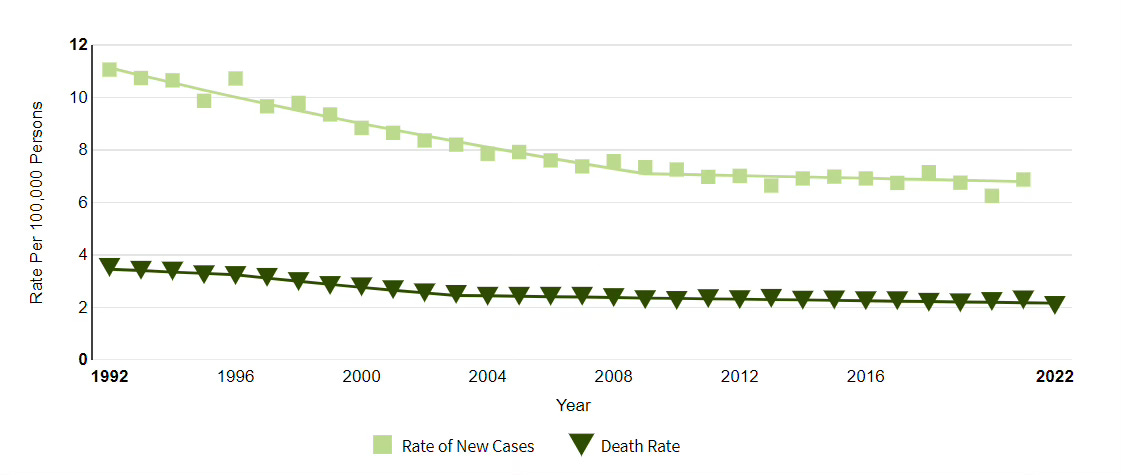
Cervical cancer cases and deaths per 100,000 population, 1992-2022
Source: National Cancer Institute LINK
The Food and Drug Administration just approved two self-collection devices (from Roche and BD) to test for human papillomavirus (HPV), the cause of most cervical cancers. This could mean in the future women will be able to test at home instead of having a pelvic examination to screen for cervical cancer. HPV tests can be performed with pap smears or can replace these to screen for cervical cancer. Pap smears are recommended at three-year intervals starting at age 21, while HPV tests are recommended at five-year intervals starting at age 25.
The FDA approval is for women to do this self-collection in a provider office, which will send the swab to a laboratory for analysis. The FDA is expected to consider approval of this device for home use later this year. The device is expected to become available in the next few months. These tests are already covered by commercial health plans and Medicare - as they use the same billing code as tests where the swab is collected by a provider.
HPV vaccines administered to adolescent girls and boys has already dramatically reduced the rate of cervical cancer in young adults, and self-testing could assist efforts to essentially eliminate cervical cancer by 2030. The National Committee on Quality Assurance (NCQA) reports that 73% of eligible members on PPO plans were up to date on screening for cervical cancer in 2022.
Implications for employers:
This approval could help lead to greater acceptance of home testing for cancer screening. FIT tests (fecal immunochemical tests) and Cologuard are already available for home testing for colorectal cancer.
Since cervical cancer screening is recommended by the US Preventive Services Task Force for women ages 21 to 65, there should be no cost sharing for those members.
Employers can continue to ask their carriers for reporting on rates of cancer screening.