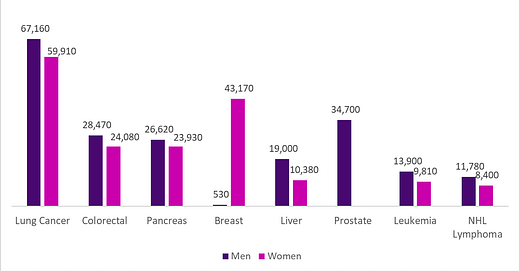
Source: Cancer.gov , 2023 LINK
Lung cancer remains the number one cancer killer among both men and women, even though we know how to prevent most lung cancer (don’t smoke!), and medical treatments for lung cancer have improved dramatically in recent years. Low dose CT scans are recommended by the US Preventive Services Task Force for those who are between 50 and 80 years of age, have smoked for 20 pack years (one year of smoking a pack a day is a “pack year,”) and have quit cigarettes within the last 15 years. As recommended preventive services, these tests are not subject to cost sharing. Nonetheless, only 5.8% of those eligible for this potentially life-saving test get it. This screening test can decrease mortality from lung cancer by 20%. The test should be repeated annually (but some people believe they need to be screened only once), and the test is used less in people of color, who die of lung cancer at a higher rate.
The American Cancer Society (ACS) has just released new guidelines, which are similar to the USPSTF guidelines except that the ACS would recommend continued screening for life - not just for the 15 years after quitting.
Implications for employers:
Employers can ask their carriers to report on lung cancer screening as a quality metric. Past or current smoking status is often not represented in medical claims, so reporting on the percentage of eligible members who are screened is difficult. An alternative is to report on the total number of low dose CT scans performed.
Those who have been nonsmokers for over 15 years (and who meet the ACS but not the USPSTF screening criteria) will likely not have cost sharing when they receive scans, since the carrier will be unlikely to know how long they have been nonsmokers. Therefore, most employers will be compliant with the new ACS guideline without taking any action.
Thanks for reading. You can find previous posts in the Employer Coverage archive
Please “like” and suggest this newsletter to friends and colleagues. Thanks!
Tomorrow: Evaluation of prescription digital therapeutics