Vitamin D screening, why biosimilars are not saving $ for employer health insurance, and quality in virtual care
September 30, 2022
Today, I’ll be covering vitamin D screening tests, which are often wasteful and has recently been denied by some health plans, why employer sponsored health plan is not benefiting so far from biosimilars, and some data about virtual care quality. There is a brief pandemic update, too.
1. Health plans starting to address overuse of vitamin D testing
Vitamin D is important. Without enough vitamin D, adults are more prone to bone fracture, and children can get rickets. Vitamin D is converted from precursor chemicals in the body by skin exposure to sunshine, and milk and infant formula are fortified with vitamin D. Deficiency of Vitamin D is rare in developed countries, and primarily affects those who are homebound. Nonetheless, Americans are frequently tested for vitamin D deficiency; vitamin D testing is one of the most frequently ordered wasteful tests.
The Endocrine Society and the American College for Clinical Pathology recommend against routine screening of vitamin D levels. Clinical guidelines suggest that vitamin D testing should be restricted to those at high risk, including those with metabolic bone disease, abnormal calcium level, malabsorption, or chronic kidney or liver disease. Researchers in Canada decreased vitamin D testing by 92% by requiring one of these diagnoses, and a team at Henry Ford Hospital in Detroit decreased their vitamin D testing by 50% by adding guidelines to their electronic medical record system.
There is much disagreement about the correct “normal” levels for vitamin D in the blood. Studies do not show a benefit from taking vitamin D supplements for a variety of ailments, from cancer to heart disease to COVID-19 to cognitive decline, even if these illnesses sometimes coincide with low vitamin D levels. Vitamin D levels are most likely a proxy for people being less healthy, as opposed to the underlying cause. Some have worried that sunscreen could lead to vitamin D deficiency, but there is no evidence of this in real-life settings. In any event, people concerned about their vitamin D level can safely take a multivitamin or a small amount of over-the-counter vitamin D supplementation. There is no reason for those at low risk of deficiency to be tested for vitamin D levels.
What does this matter to employers?
Some national and regional health plans are now denying claims for vitamin D assays unless they are accompanied by a diagnosis that makes the test appropriate. This is an example of “value-based insurance design,” where there is higher member cost sharing (in this case no coverage) for low value care. Employers should support this evidence-based approach, which will save substantial resources while still allowing appropriate testing for high-risk individuals. Specifically, employers can ask if their health plans have a policy to prevent reimbursement for unnecessary vitamin D tests, and ask for reporting on their spending on these tests.
2. Commercial health plans lag Medicare in adoption of biosimilars
Biologic drugs have helped us make dramatic progress in treating inflammatory diseases including cancer, multiple sclerosis, rheumatoid arthritis, inflammatory bowel disease, and psoriasis. These drugs are generally given by injection and require yeast or other living organisms for manufacturing - so they are expensive.
Biologic drugs represent the majority of the increase in pharmacy costs - and we have been hopeful for years that biosimilars, biologic drugs with no meaningful differences from the brand name drug, would help keep costs down. The most widely prescribed biologic drug, Humira, went off patent in 2016 and will face a biosimilar competitor in January, 2023, and multiple biosimilar competitors by next summer. Humira cost about $13,000 annually when it was first marketed in 2003, and now costs about $77,000 a year.
Biosimilars have had relatively little economic impact in the US compared to other countries. For instance, there are 55 biosimilars in use in Europe, while only 15 have been licensed in the US. Biosimilars in Europe are approved for marketing shortly after patent expiration without extended patent fights, and quickly attain high market share. In the US, brand name biologic drugs have been more successful at maintaining their market share in part due to rebates and other peculiarities of the US market, like patent litigation. In Canada for example, 6 biosimilars are available for Humira. As of December 2021, 81% of patients had switched to the biosimilar.
Researchers at the Health Care Cost Institute used their database of claims for about 50 million people covered by employer sponsored health insurance to evaluate the impact of a biosimilar on prices for Medicare and employer sponsored health insurance. The researchers studied Neupogen, a drug which helps increase the supply of white blood cells for those getting chemotherapy, and Zarxio, the corresponding biosimilar. While the introduction of a biosimilar lowered costs substantially for Medicare, the impact on costs for employer sponsored health insurance was quite modest.
Cost of Neupogen (brand name) and Zarxio (biosimilar) 2015-2020
Source: Health Care Cost Institute, 9/22/22 LINK
Why did Medicare see costs of drug acquisition drop dramatically? Likely because utilization of the biosimilar was high in Medicare, while rebate arrangements helped brand name drugs retain their market share in employer sponsored health insurance. (The researchers did not publish utilization numbers.) Biosimilars can only save money if they are widely used.
Employers can encourage their pharmacy benefit managers to promote the use of biosimilars, and discourage mechanisms that might delay conversion from brand name biologics. The FDA has approved seven biosimilars for Humira for mid-2023 (with others expected later in the year). Past experience has shown the large decreases in overall acquisition costs when there are multiple generics or biosimilars available. The opportunity for meaningful savings on this very effective therapy could be large, but we should watch carefully to be sure that employer sponsored health insurance will gain economic benefits from biosimilars.
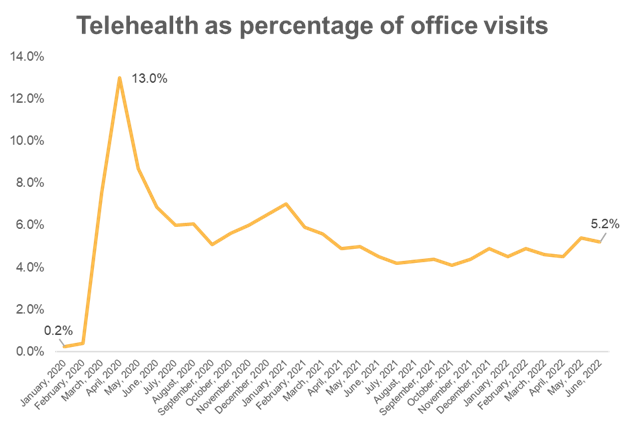
3. Telehealth appears not to diminish the quality of primary care
Virtual visits peaked at 13-15% of all office visits in Spring, 2020, and remain about 5% of all office visits today. Prior to the pandemic, virtual visits represented less than 1% of all office visits.
Source: Fair Health 2022 LINK
It’s at least somewhat reassuring to see data from JAMA Network Open demonstrating that many measures of quality appear to be well preserved in those who had a telemedicine visit for primary care. Researchers reviewed medical claims and electronic health records of about a half million people in Pennsylvania and Maryland, a fifth of whom had at least one telemedicine visit. Those who had telemedicine visits were statistically significantly more likely to have had appropriate screening tests and vaccinations, and statistically significantly more likely to have had a normal blood pressure. There were four medication-based measures where those individuals without a telemedicine visit scored better (statins and antiplatelet drugs for those with heart disease, statins for diabetics, and inappropriate use of respiratory antibiotics).
This suggests that telemedicine providers overprescribed antibiotics for upper respiratory infections, and suggests that primary care providers might do a better job refining prescribing for patients with chronic disease who are seen in person. Those who were seen in telemedicine continued to have full access to in-person care, and we should not conclude that quality measures would be sustained in a plan where members were required or strongly urged to use virtual care.
Employers should continue to carefully monitor the utilization of virtual care. The combination of access to virtual and in-person visits is likely optimal from a quality perspective. Virtual visits should save money, as they require less time and resources, and they rarely result in ancillary tests. But “downshifting” in medical care doesn’t always save money. For example, researchers determined that retail clinics increase total health care costs, as 58% of visits were not substitutes for in-person visits, but were rather visits that otherwise would not have occurred.
4. COVID-19 Update
- Pfizer and BioNTech have filed for FDA approval for its bivalent COVID-19 vaccine for 5-11 year olds, and Moderna has filed for Food and Drug Administration approval for its bivalent COVID-19 vaccine for those ages 6-17. Both are expected to be approved in the coming weeks, adding to our ability to prevent large spikes over the fall and winter. However, the rate of initial two-dose vaccination of children ages 5-11 remains under a third.
- An upcoming report from France describes laboratory evidence of 188 cases of recurrent COVID-19 infection with different strains of Omicron. About one in four of these infections was within 90 days, and one in seven was within 60 days. This suggests that recurrent infections can happen more rapidly than we had previously thought.
- COVID vaccination continues to be associated with substantially lower rates of infection. Here is the most recent summary from the Centers for Disease Control and Prevention(CDC), showing a 2-fold difference in infection for vaccinated people compared to unvaccinated people. There is a 12-fold difference in age-adjusted risk of death (not pictured).